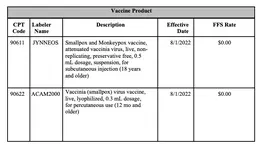
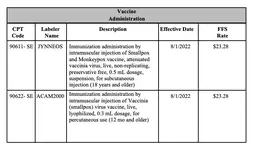
The CPT Editorial Panel has approved the addition of code 90622 to identify vaccinia (smallpox) virus vaccine product; and the addition of code 90611 to identify monkeypox and smallpox virus vaccine product.
90611: Smallpox and monkeypox vaccine, attenuated vaccinia virus, live, non-replicating, preservative free, 0.5 mL dosage, suspension, for subcutaneous use
90622: Vaccinia (smallpox) virus vaccine, live, lyophilized, 0.3 mL dosage, for percutaneous use
What is Monkeypox?
 www.ama-assn.org
www.ama-assn.org
CPT Assistant for Monkeypox
 www.ama-assn.org
www.ama-assn.org
90611: Smallpox and monkeypox vaccine, attenuated vaccinia virus, live, non-replicating, preservative free, 0.5 mL dosage, suspension, for subcutaneous use
90622: Vaccinia (smallpox) virus vaccine, live, lyophilized, 0.3 mL dosage, for percutaneous use
Laboratory test code | |
87593 | Infectious agent detection by nucleic acid (DNA or RNA); orthopoxvirus (eg, monkeypox virus, cowpox virus, vaccinia virus), amplified probe technique, each |
What is Monkeypox?
Mpox
Mpox is a rare disease caused by infection with the Mpox virus. In the United States, there is currently evidence of person-to-person disease transmission. CDC is urging clinicians in the U.S. to be alert for patients who have rash illnesses consistent with Mpox. The AMA keeps you current on the...
CPT Assistant for Monkeypox
Orthopoxvirus and monkeypox coding & guidance
Find CPT® codes for reporting orthopoxvirus and monkeypox testing and immunizations.
Last edited: