Prescription drug management is based on documented evidence that the physician has evaluated medications as part of a service that is provided. Physicians should make a direct connection between the medication that is prescribed to the patient and the work that was performed on the day of the clinic visit.
How to determine Rx Management:
Prescription drug management as long as the documentation clearly shows decision-making took place regarding those medications count.
The mere presence of prescription drugs does not necessarily qualify for moderate complexity. CMS has indicated that writing a prescription for a seven- or 10-day supply of an antibiotic is not considered to be a moderate level of complexity. At least one Blue Cross company has indicated that prescription drug management involves more than the use of prescription drugs. It may mean a change in regimen, the addition of an agent, or the worsening of a problem. In other words, any prescription is not a guarantee that a payer will see this your way.
Consider the entry in the first column of the table, Presenting Problem, under low level decision making. It says "acute uncomplicated illness or injury; e.g., cystitis, allergic rhinitis, simple sprain." Does the new problem that you were describing fit into this category? If so, you might be more accurate - as you indicated - with the low level decision making associated with a 99213.
From the AMA
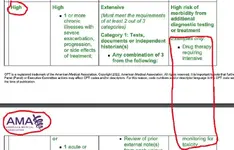
“Drug therapy requiring intensive monitoring for toxicity: A drug that requires intensive monitoring is a therapeutic agent that has the potential to cause serious morbidity or death. The monitoring is performed for assessment of these adverse effects and not primarily for assessment of therapeutic efficacy. The monitoring should be that which is generally accepted practice for the agent, but may be patient specific in some cases. Intensive monitoring may be long-term or short term. Long-term intensive monitoring is not less than quarterly. The monitoring may be by a lab test, a physiologic test or imaging. Monitoring by history or examination does not qualify. The monitoring affects the level of medical decision making in an encounter in which it is considered in the management of the patient. Examples may include monitoring for a cytopenia in the use of an antineoplastic agent between dose cycles or the short-term intensive monitoring of electrolytes and renal function in a patient who is undergoing diuresis. Examples of monitoring that does not qualify include monitoring glucose levels during insulin therapy as the primary reason is the therapeutic effect (unless severe hypoglycemia is a current, significant concern); or annual electrolytes and renal function for a patient on a diuretic as the frequency does not meet the threshold.”
Z51.81
Encounter for therapeutic drug level monitoring
Approximate Synonyms
Antihypertensive agent surveillance
Medication surveillance, antihypertensive
Monitoring growth hormone therapy
Monitoring growth hormone therapy done
Therapeutic drug monitoring
Therapeutic drug monitoring done
Table of Risk
How does "drug therapy requiring intensive monitoring" differ from "prescription drug management?"
Per the CPT definition, "drug therapy requiring intensive monitoring for toxicity" is for a drug requiring intensive monitoring which is a therapeutic agent with the potential to cause serious morbidity or death. The monitoring is performed for assessment of these adverse effects and not primarily for assessment of therapeutic efficacy.
The monitoring should be that which is generally accepted practice for the agent but may be patient-specific in some cases. Intensive monitoring may be long-term or short-term. Long-term intensive monitoring is not less than quarterly. The monitoring may be by a lab test, a physiologic test or imaging. Monitoring by history or examination does not qualify. The monitoring affects the level of medical decision-making in an encounter in which it is considered in the management of the patient. Examples may include monitoring for cytopenia in the use of an antineoplastic agent between dose cycles or the short-term intensive monitoring of electrolytes and renal function in a patient who is undergoing diuresis.
"Prescription drug management" is documented evidence the provider has evaluated the patient's medications as part of an E/M service. This may be a prescription being written or discontinued, or a decision to maintain a current medication or dosage. However, simply listing current medications is not considered "prescription drug management."
1. Monitoring by history or examination does not qualify.
2. Monitoring glucose levels during insulin therapy. The primary reason for the monitoring is the therapeutic effect (unless severe hypoglycemia is a current, significant concern), not toxicity levels.
3. Annual monitoring of electrolytes for any reason, as the frequency does not meet the threshold for intensive monitoring.
4. Annual renal function panel for a patient on a diuretic, as the frequency does not meet the threshold for intensive monitoring.

 www.terryfletcher.net
www.terryfletcher.net
 www.physicianspractice.com
www.physicianspractice.com

 rtwelter.com
rtwelter.com
How to determine Rx Management:
- Did it require prescriptive authority?
- Is the provider of record managing the Rx?
- A prescription drug that the practitioner is evaluating the appropriateness of using for the patient; and/or continuing to prescribe for the patient.
- Documentation of the prescription drug(s) that are being considered and the reason why they are being considered.
- Documentation of a practitioner’s decision to discontinue a prescription drug or to adjust the current dosage relative to changes in a patient’s condition.
- The patient’s condition, possible adverse effects, potential benefits, etc. of the patient using this prescription drug.
Prescription drug management as long as the documentation clearly shows decision-making took place regarding those medications count.
The mere presence of prescription drugs does not necessarily qualify for moderate complexity. CMS has indicated that writing a prescription for a seven- or 10-day supply of an antibiotic is not considered to be a moderate level of complexity. At least one Blue Cross company has indicated that prescription drug management involves more than the use of prescription drugs. It may mean a change in regimen, the addition of an agent, or the worsening of a problem. In other words, any prescription is not a guarantee that a payer will see this your way.
Consider the entry in the first column of the table, Presenting Problem, under low level decision making. It says "acute uncomplicated illness or injury; e.g., cystitis, allergic rhinitis, simple sprain." Does the new problem that you were describing fit into this category? If so, you might be more accurate - as you indicated - with the low level decision making associated with a 99213.
From the AMA
“Drug therapy requiring intensive monitoring for toxicity: A drug that requires intensive monitoring is a therapeutic agent that has the potential to cause serious morbidity or death. The monitoring is performed for assessment of these adverse effects and not primarily for assessment of therapeutic efficacy. The monitoring should be that which is generally accepted practice for the agent, but may be patient specific in some cases. Intensive monitoring may be long-term or short term. Long-term intensive monitoring is not less than quarterly. The monitoring may be by a lab test, a physiologic test or imaging. Monitoring by history or examination does not qualify. The monitoring affects the level of medical decision making in an encounter in which it is considered in the management of the patient. Examples may include monitoring for a cytopenia in the use of an antineoplastic agent between dose cycles or the short-term intensive monitoring of electrolytes and renal function in a patient who is undergoing diuresis. Examples of monitoring that does not qualify include monitoring glucose levels during insulin therapy as the primary reason is the therapeutic effect (unless severe hypoglycemia is a current, significant concern); or annual electrolytes and renal function for a patient on a diuretic as the frequency does not meet the threshold.”
Z51.81
Encounter for therapeutic drug level monitoring
Approximate Synonyms
Antihypertensive agent surveillance
Medication surveillance, antihypertensive
Monitoring growth hormone therapy
Monitoring growth hormone therapy done
Therapeutic drug monitoring
Therapeutic drug monitoring done
- Therapeutic agent that has the potential to cause serious morbidity or death
- Monitoring is performed for assessment of adverse effects and not primarily for assessment of therapeutic efficacy
- May be patient-specific in some cases
- Long term or short term
- Not less than quarterly
- Laboratory test, physiologic test or imaging
- History and exam does not qualify
- Cytotoxic chemotherapy treatment
- The table below is a list of examples of drugs that are approved for monitoring for toxicity – when used for the specific treatment listed (this is not an all-inclusive list)
Table of Risk
Drug Category | ||
Cardiac | Digoxin, Digitoxin, Quinidine, Procainamide, Amiodarone | Congestive heart failure, angina, arrhythmias |
Anticoagulants | Coumadin and intravenous Heparin drip (Heparin must be provided in the hospital setting) | Prevention of thrombosis and thromboembolisms |
Antiepileptic | Phenobarbital, Phenytoin, Valproic Acid, Carbamazepine, Ethosuximide, sometimes Gabapentin, Lamotrigine | Epilepsy, prevention of seizures, sometimes to stabilize moods |
Bronchodilators | Theophylline, Caffeine | Asthma, chronic obstructive pulmonary disorder (COPD), neonatal apnea |
Immunosuppressant | Cyclosporine, Tacrolimus, Sirolimus, Mycophenolate Mofetil, Azathioprine | Prevent rejection of transplanted organs, autoimmune disorders |
Anti-Cancer | All Cytotoxic agents | Multiple malignancies |
Psychiatric | Lithium, Valproic Acid, some antidepressants (Imipramine, Amitriptyline, Nortriptyline, Doxepin, Desipramine) | Bipolar disorder (manic depression), depression |
Protease Inhibitors | Indinavir, Ritonavir, Lopinavir, Saquinavir, Atazanavir, Nelfinavir | HIV/AIDS |
Antibiotics | Aminoglycosides (Gentamicin, Tobramycin, Amikacin) Vancomycin, Chloramphenicol, Cubicin, Zyvox | Infections with bacteria that are resistant to less toxic antibiotics |
Insulin/Anti-Diabetic | Intravenous Insulin drip | Hyperglycemia |
Erythropoiesis-Stimulating Agents (ESA) | Procrit and Epogen (Epoetin Alfa) and Aranesp (Darbepoetin Alfa) | Anemia |
How does "drug therapy requiring intensive monitoring" differ from "prescription drug management?"
Per the CPT definition, "drug therapy requiring intensive monitoring for toxicity" is for a drug requiring intensive monitoring which is a therapeutic agent with the potential to cause serious morbidity or death. The monitoring is performed for assessment of these adverse effects and not primarily for assessment of therapeutic efficacy.
The monitoring should be that which is generally accepted practice for the agent but may be patient-specific in some cases. Intensive monitoring may be long-term or short-term. Long-term intensive monitoring is not less than quarterly. The monitoring may be by a lab test, a physiologic test or imaging. Monitoring by history or examination does not qualify. The monitoring affects the level of medical decision-making in an encounter in which it is considered in the management of the patient. Examples may include monitoring for cytopenia in the use of an antineoplastic agent between dose cycles or the short-term intensive monitoring of electrolytes and renal function in a patient who is undergoing diuresis.
"Prescription drug management" is documented evidence the provider has evaluated the patient's medications as part of an E/M service. This may be a prescription being written or discontinued, or a decision to maintain a current medication or dosage. However, simply listing current medications is not considered "prescription drug management."
Monitoring that does not qualify
Examples of monitoring that does not qualify includes:1. Monitoring by history or examination does not qualify.
2. Monitoring glucose levels during insulin therapy. The primary reason for the monitoring is the therapeutic effect (unless severe hypoglycemia is a current, significant concern), not toxicity levels.
3. Annual monitoring of electrolytes for any reason, as the frequency does not meet the threshold for intensive monitoring.
4. Annual renal function panel for a patient on a diuretic, as the frequency does not meet the threshold for intensive monitoring.



Prescription Drug Management: The Moderate Risk element of E/M Coding - Terry Fletcher Consulting, Inc.
Appropriate documentation of prescription drug management continues to be an opportunity for many physicians. Doctors need to know that simply adding the current medication list to the progress note is not adequate to include in the level of risk of a moderate visit. Prescription drug management...
Prescription Drug Management
Does prescribing a new medication qualify as a moderate level decision making?



